
SL Paper 3
The process of converting heat to electricity is limited by its thermal (Carnot) efficiency.
\({\text{Thermal efficiency}} = \frac{{{\text{temp. of steam at source (K) }}-{\text{ temp. heat sink (K)}}}}{{{\text{temp. of steam at source (K)}}}} \times 100\)
Calculate the thermal efficiency of a steam turbine supplied with steam at 540°C and using a river as the choice of sink at 23 °C.
Power plants generating electricity by burning coal to boil water operate at approximately 35% efficiency.
State what this means and suggest why it is lower than the thermal efficiency.
Chemical energy from redox reactions can be used as a portable source of electrical energy. A hybrid car uses a lithium ion battery in addition to gasoline as fuel.
(i) Calculate the specific energy of the lithium ion battery, in MJ kg−1, when 80.0 kg of fuel in the battery releases 1.58 × 107 J. Use section 1 of the data booklet.
(ii) The specific energy of gasoline is 46.0 MJ kg−1. Suggest why gasoline may be considered a better energy source than the lithium ion battery based on your answer to part (a) (i).
(i) The energy density of gasoline is 34.3 MJ dm−3. Calculate the volume of gasoline, in dm3, that is equivalent to the energy in 80.0 kg of fuel in the lithium ion battery. Use section 1 of the data booklet.
(ii) The efficiency of energy transfer by this lithium ion battery is four times greater than that of gasoline. Determine the distance, in km, the car can travel on the lithium ion battery power alone if the gasoline-powered car uses 1.00 dm3 gasoline to travel 32.0 km.
The sun is the main source of energy used on earth.
One fusion reaction occurring in the sun is the fusion of deuterium, \({}_1^2H\), with tritium, \({}_1^3H\), to form helium, \({}_2^4He\). State a nuclear equation for this reaction.
Explain why this fusion reaction releases energy by using section 36 of the data booklet.
State the technique used to show that the sun is mainly composed of hydrogen and helium.
Coloured molecules absorb sunlight. Identify the bonding characteristics of such molecules.
In the 20th Century, both fission and fusion were considered as sources of energy but fusion was economically and technically unattainable.
Compare and contrast fission and fusion in terms of binding energy and the types of nuclei involved.
Suggest two advantages that fusion has over fission.
The amount of 228Ac in a sample decreases to one eighth \(\left( {\frac{1}{8}} \right)\) of its original value in about 18 hours due to β-decay. Estimate the half-life of 228Ac.
One method of comparing fuels is by considering their specific energies.
Calculate the specific energy of octane, C8H18, in kJ kg–1 using sections 1, 6 and 13 of the data booklet.
A typical wood has a specific energy of 17 × 103 kJ kg–1. Comment on the usefulness of octane and wood for powering a moving vehicle, using your answer to (a).
If you did not work out an answer for (a), use 45 × 103 kJ kg–1 but this is not the correct answer.
State the name of one renewable source of energy other than wood.
Carbon dioxide is a product of the combustion of petrol.
Explain the molecular mechanism by which carbon dioxide acts as a greenhouse gas.
Discuss the significance of two greenhouse gases, other than carbon dioxide, in causing global warming or climate change.
One suggestion for the reduction of carbon footprints is the use of biofuels, such as vegetable oils, as a substitute for petroleum based fuels.
Outline the major technical problem affecting the direct use of vegetable oils as fuels in internal combustion engines and the chemical conversion that has overcome this.
State the formula of a fuel that might be produced from the vegetable oil whose formula is shown.
Outline why biofuels are considered more environmentally friendly, even though they produce more carbon dioxide per kJ of energy than petroleum based fuels.
Crude oil is a useful energy resource.
Outline two reasons why oil is one of the world’s significant energy sources.
Formulate an equation for the cracking of C16H34 into two products with eight carbon atoms each.
Identify, giving a reason, which product in (b)(i) could be used in petrol (gasoline).
Outline how higher octane fuels help eliminate “knocking” in engines.
The performance of hydrocarbons as fuels can be improved by catalytic reforming.
Outline how catalytic reforming increases a fuel’s octane rating.
Greenhouse gases absorb infrared radiation.
Identify one naturally occurring greenhouse gas, other than carbon dioxide or water vapour, and its natural source.
Formulate an equation that shows how aqueous carbon dioxide produces hydrogen ions, H+(aq).
The concentrations of oxygen and nitrogen in the atmosphere are much greater than those of greenhouse gases. Outline why these gases do not absorb infrared radiation.
A link between the combustion of fossil fuels and an increase in the temperature of the Earth’s atmosphere was proposed over a century ago.
Suggest why it is only in recent years that specific predictions of the future effects of fossil fuel combustion have been made.
Carbon dioxide has two different bond stretching modes illustrated below.

Predict, with an explanation, whether these stretching modes will absorb infrared radiation.
Outline, giving the appropriate equation(s), how increasing levels of carbon dioxide will affect the pH of the oceans.
Many combustion processes also release particulate matter into the atmosphere. Suggest, giving your reason, how this might affect the temperature of the Earth’s surface.
Although fossil fuels are considered significant sources of energy, the energy conversion associated with the production of electricity is a very inefficient process, often in the region of only 40% of total possible energy conversion.
Fuel cells provide a much more efficient process, often with a 70% conversion factor.
State the energy change conversion involved in a fuel cell.
(i) Identify the two half-equations that take place at the positive electrode (cathode) and negative electrode (anode) in a hydrogen-oxygen fuel cell with an alkaline electrolyte.
Positive electrode (cathode) half-equation:
Negative electrode (anode) half-equation:
(ii) State the overall reaction, identifying the states of all species involved.
(iii) Outline the function of the thin polymer membrane used in the corresponding hydrogen-oxygen fuel cell with an acidic electrolyte.
(iv) Other than cost, state one disadvantage of a fuel cell.
Catalysts may be homogeneous or heterogeneous.
Distinguish between homogeneous and heterogeneous catalysts.
Discuss two factors which need to be considered when selecting a catalyst for a particular chemical process.
Identify the catalyst used in the catalytic cracking of long chain hydrocarbons and state one other condition needed.
State an equation for the catalytic cracking of the straight chain hydrocarbon pentadecane, \({{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{32}}}}\), to produce two products with similar masses.
Much of our energy needs are still provided by the refined products of crude oil.
“Knocking” in an automobile (car) engine can be prevented by increasing the octane number of the fuel. Explain, including an equation with structural formulas, how heptane, C7H16, could be chemically converted to increase its octane number.
Many like to refer to our “carbon footprint”. Outline one difficulty in quantifying such a concept.
Climate change or global warming is a consequence of increased levels of carbon dioxide in the atmosphere. Explain how the greenhouse effect warms the surface of the earth.
Outline how water and carbon dioxide absorb infrared radiation.
The increased concentration of carbon dioxide in the atmosphere is thought to result from the increased combustion of fossil fuels such as petroleum.
Identify an element, other than carbon and hydrogen, found at significant concentrations in fossil fuels.
Petroleum contains many hydrocarbons. Explain how these are separated by fractional distillation.
Determine the specific energy and energy density of petrol (gasoline), using data from sections 1 and 13 of the data booklet. Assume petrol is pure octane, C8H18. Octane: molar mass = 114.26 g mol−1, density = 0.703 g cm−3.
Outline why the energy available from an engine will be less than these theoretical values.
Carbon dioxide, methane and chlorofluorocarbons (CFCs) are well known greenhouse gases. Nitrogen trifluoride, \({\text{N}}{{\text{F}}_{\text{3}}}\), is thousands of times more effective at warming the atmosphere than an equal mass of carbon dioxide. \({\text{N}}{{\text{F}}_{\text{3}}}\) can be used in the manufacture of computer chips and thin-film solar photovoltaic cells.
Identify two greenhouse gases not mentioned above. One of the gases that you identify should contain a nitrogen atom. For each gas, state its source.
Greenhouse gas 1:
Source:
Greenhouse gas 2:
Source:
The methane produced by sheep and cows can contribute to global warming. In Australia, it is considered that sheep and cows produce approximately 14% of the country’s total greenhouse emissions. Explain how this methane is formed.
The following graph shows the annual increase in the concentration of atmospheric carbon dioxide recorded at Mauna Loa, Hawaii.
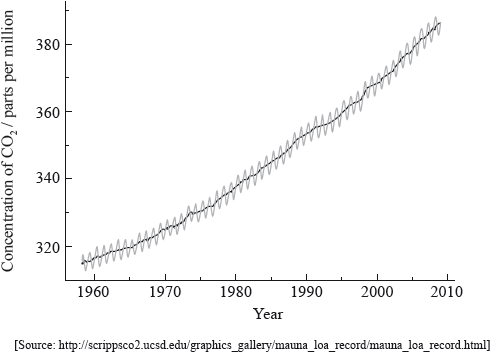
Explain why the graph is not smooth but involves annual fluctuations (shown in grey).
State one effect of global warming.
Nuclear power is another source of energy.
Compare and contrast the process of nuclear fusion with nuclear fission.
Dubnium-261 has a half-life of 27 seconds and rutherfordium-261 has a half-life of 81 seconds.
Estimate what fraction of the dubnium-261 isotope remains in the same amount of time that \(\frac{3}{4}\) of rutherfordium-261 decays.
Nuclear fission of 235U is one source of electrical energy that has a minimal carbon footprint.
Natural uranium needs to be enriched to increase the proportion of 235U. Suggest a technique that would determine the relative abundances of 235U and 238U.
Explain how 235U fission results in a chain reaction, including the concept of critical mass.
Suggest one reason why there is opposition to the increased use of nuclear fission reactors.
One method of producing biodiesel is by a transesterification process.
Deduce the equation for the transesterification reaction of pentyl octanoate, C7H15COOC5H11, with methanol.
Outline why the ester product of this reaction is a better diesel fuel than pentyl octanoate.
Petroleum (mineral oil) can be used either as a fuel or a chemical feedstock.
Name two fuels that are obtained from petroleum.
Describe one environmental problem that can result from the combustion of these fuels in the internal combustion engine and identify the specific combustion product responsible.
Plastic litter is an environmental problem that results from the use of petroleum as a chemical feedstock. Identify the property of plastics that is responsible for this.
One product that is made from crude oil is the chemical feedstock that can be used to synthesize commercial liquid-crystal displays. Discuss the properties that a substance must have to make it suitable for use as a liquid-crystal display.
Nuclear reactions transform one nuclide into another. Fission, splitting a large nucleus into two smaller nuclei, releases vast amounts of energy.
(i) Explain why fusion, combining two smaller nuclei into a larger nucleus, releases vast amounts of energy. Use section 36 of the data booklet.
(ii) Outline one advantage of fusion as a source of energy.
Radioactive phosphorus, 33P, has a half-life of 25.3 days.
(i) Calculate 33P decay constant λ and state its unit. Use section 1 of the data booklet.
(ii) Determine the fraction of the 33P sample remaining after 101.2 days.
Carbon is produced by fusion reactions in stars.
The main fusion reaction responsible for the production of carbon is:
X + \(_2^4{\text{He}} \to _{\;6}^{12}{\text{C}}\)
Outline how the spectra of light from stars can be used to detect the presence of carbon.
Deduce the identity of X.
Outline why this reaction results in a release of energy.
Nuclear fusion reactors are predicted to become an important source of electrical energy in the future. State two advantages of nuclear fusion over nuclear fission.
Thermal cracking, catalytic cracking and steam cracking are all used to convert alkane molecules into smaller molecules. Identify which one of the three types of cracking is used to crack a hexane molecule, C6H14, into propane and an alkene molecule, and state the equation involved.
Auto-ignition of hydrocarbon fuel in a car engine causes “knocking”. The tendency of a fuel to knock depends on its molecular structure.
Discuss how the octane number changes with the molecular structure of the alkanes.
Catalytic reforming and cracking reactions are used to produce more efficient fuels. Deduce the equation for the conversion of heptane to methylbenzene.
Fuel cells convert chemical energy directly into electrical energy that can be used in applications ranging from spacecraft to remote weather stations.
Describe the composition of the electrodes in a hydrogen-oxygen fuel cell.
State the half-equation at each electrode in the hydrogen-oxygen alkaline cell.
Positive electrode (cathode):
Negative electrode (anode):
Hexane, C6H14, is not a suitable fuel for internal combustion engines as it has a tendency to auto-ignite, a cause of “knocking”.
(i) Hexane can be converted to different organic products in a reforming process. Identify one of these products.
(ii) Suggest why the product in (a)(i) has a lesser tendency to auto-ignite than hexane.
(i) Octane, C8H18, can undergo complete combustion under suitable conditions. Calculate the specific energy of octane, in kJg−1, using sections 1, 6 and 13 of the data booklet.
(ii) The specific energy of ethanol is 29.7kJg−1. Evaluate the addition of ethanol to octane (or its isomers) for use as a fuel in motor vehicles, giving one advantage and one disadvantage.
Advantage:
Disadvantage:
Coal can be heated with steam to produce synthetic natural gas. Formulate an equation to show the formation of methane, CH4(g), from coal, C(s), and steam, H2O(g).
Although crude oil is considered an extremely important energy source, it cannot be used directly as a resource.
Suggest why crude oil needs to be refined before it can be used.
Thermal cracking, catalytic cracking and steam cracking can all be used to convert molecules of alkanes into alkenes.
(i) State the type of cracking which can be used to crack ethane into ethene, the chemical equation for the process and one reaction condition required.
Type of cracking:
Chemical equation:
Reaction condition:
(ii) Suggest one use for the other product formed in this reaction in addition to ethene.
Fuel cells and rechargeable batteries are both convenient ways of providing portable electric power.
Compare fuel cells and rechargeable batteries giving one similarity and one difference.
Similarity:
Difference:
One common type of rechargeable cell is the nickel–cadmium (NiCad) battery. For each terminal of this battery state the initial and final oxidation number of the element when the cell is delivering a current. Hence deduce which electrode is acting as the anode and which the cathode.

A common type of fuel cell uses hydrogen and oxygen with an acidic electrolyte. State the half-equations for the reactions at the two electrodes.
Positive electrode:
Negative electrode:
The electrodes of fuel cells and rechargeable batteries have a feature in common with heterogeneous catalysts. Identify this feature and state why it is important for them to work efficiently.
Carbon dioxide and water vapour are greenhouse gases produced by the combustion of fossil fuels.
Explain the effect of the increasing concentration of atmospheric carbon dioxide on the acidity of oceans.
(i) Describe the changes that occur at the molecular level when atmospheric carbon dioxide gas absorbs infrared radiation emitted from the Earth’s surface.
(ii) Other than changes to the acidity of oceans, suggest why the production of carbon dioxide is of greater concern than the production of water vapour.
The greenhouse effect maintains the earth’s temperature, which makes the planet habitable. However, over the last 100 years the average temperature of the earth has increased by almost 1 °C. Most climate scientists believe this warming is due to increased levels of greenhouse gases in the atmosphere.
Two of the major greenhouse gases in the atmosphere are methane and carbon dioxide. State two other major greenhouse gases.
Discuss which two gases from the four gases in part (a) are the most significant for global warming.
Discuss two effects of global warming.
Coal is often converted to liquid hydrocarbon fuels through initial conversion to carbon monoxide and hydrogen.
State how these gases are produced, giving the appropriate equation(s).
Outline how the carbon monoxide is then converted to a hydrocarbon fuel.
State the characteristics and sources of low-level nuclear waste.
The disposal of nuclear waste in the sea is now banned in many countries. Discuss one method of storing high-level nuclear waste and two problems associated with it.
Landfill sites are used to dispose of about 90% of the world’s domestic waste, but incineration is being increasingly used in some countries.
Suggest why some biodegradable plastics do not decompose in landfill sites.
High-level and low-level wastes are two types of radioactive waste. Compare the half-lives and the methods of disposal of the two types of waste.
the oil industry surplus long-chain hydrocarbons are converted into shorter, more useful hydrocarbons by various kinds of cracking.
State whether each of the following are examples of homogeneous or heterogeneous catalysis.
Steam cracking:
Catalytic cracking:
Hydrocracking:
Carbon dioxide, CO2, is a greenhouse gas. Outline, in molecular terms, how carbon dioxide molecules absorb infrared radiation.
The temperature of the Earth is increasing. There is considerable scientific evidence to suggest this is due to an increase in the concentration of greenhouse gases as a result of human activity.
Explain how this enhanced greenhouse effect causes the average temperature of the Earth to increase.
Compare the contributions of carbon dioxide and methane to the enhanced greenhouse effect.
Lead–acid batteries are heavy. Much lighter rechargeable cells are nickel–cadmium batteries used in electronic equipment.
A fuel cell can be made using an electrolyte of aqueous sodium hydroxide with porous electrodes which allow the passage of water, hydrogen and oxygen. State the equations for the reactions that occur at the positive and negative electrodes.
(+) electrode (cathode):
(–) electrode (anode):
Electricity can also be generated from a lead–acid storage battery. The electrolyte is a solution of sulfuric acid and the electrodes are made of lead and lead(IV) oxide. State the equations for the reactions that occur at the positive and negative electrodes.
(+) electrode (cathode):
(–) electrode (anode):
(i) Explain why fuel cells are less damaging to the environment than nickel–cadmium batteries.
(ii) Other than cost, state one major difference between fuel cells and nickel–cadmium cells.
State the half-equations for the reactions taking place at the negative electrode (anode) and the positive electrode (cathode) in an alkaline hydrogen-oxygen fuel cell.
Negative electrode (anode):
Positive electrode (cathode):
A different type of cell has the half-equation below.
\[{\text{L}}{{\text{i}}^ + }{\text{(polymer)}} + {\text{Mn}}{{\text{O}}_2}{\text{(s)}} + {{\text{e}}^ - } \to {\text{LiMn}}{{\text{O}}_2}{\text{(s)}}\]
Identify this type of cell.
Both fuel cells and rechargeable batteries offer great potential for the future. Compare these two power sources.
Suggest two problems associated with using hydrogen gas in a fuel cell.
The high activity of lithium metal leads to the formation of an oxide layer on the metal which decreases the contact with the electrolyte in a battery.
Describe how this is overcome in the lithium-ion battery.
Describe the migration of ions taking place at the two electrodes in the lithium-ion battery when it produces electricity.
Anode (–):
Cathode (+):
Discuss one similarity and one difference between fuel cells and rechargeable batteries.
Similarity:
Difference:
Carbon dioxide, methane, ozone, chlorofluorocarbons (CFCs) and water are examples of greenhouse gases.
Describe how these gases contribute to the greenhouse effect.
(i) Identify by chemical formula one other greenhouse gas not mentioned above.
(ii) State the source of this gas.
Many scientists claim that global warming is associated with the increasing concentration of greenhouse gases in the atmosphere. Other than temperature change, state two effects of global warming.
It is now widely accepted that the increased production of carbon dioxide is leading to global warming.
Describe how carbon dioxide acts as a greenhouse gas.
Discuss the influence of increasing amounts of greenhouse gases on the environment.
(a) Explain why the nitrogen molecule, \({{\text{N}}_2}\), does not absorb infrared radiation.
(b) Describe two vibrations in the water molecule that absorb infrared radiation.
Rechargeable nickel-cadmium batteries are used in portable electrical equipment and emergency lighting.
The discharge process can be summarized by the equation below.
\[{\text{2NiO(OH)(s)}} + {\text{Cd(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{2Ni(OH}}{{\text{)}}_{\text{2}}}{\text{(s)}} + {\text{Cd(OH}}{{\text{)}}_{\text{2}}}{\text{(s)}}\]
State the change in oxidation number of the cadmium and deduce if it is acting as the positive or negative electrode during the discharge process.
Identify a physical property of Cd(OH)2 which allows this process to be reversed and the battery recharged.
Cracking is the process by which long-chain alkanes found in oil are broken down into smaller molecules.
The following reaction occurs during the cracking of tetradecane, \({{\text{C}}_{{\text{14}}}}{{\text{H}}_{{\text{30}}}}\).
\[{{\text{C}}_{14}}{{\text{H}}_{30}}{\text{(g)}} \to {{\text{C}}_{10}}{{\text{H}}_{22}}{\text{(g)}} + {\text{2}}{{\text{C}}_2}{{\text{H}}_4}{\text{(g)}}\]
Suggest a use for each of the products formed in the reaction.
\({{\text{C}}_{{\text{10}}}}{{\text{H}}_{{\text{22}}}}\):
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\):
State the main type of product obtained from steam cracking.
Catalytic cracking uses silica as a heterogeneous catalyst. Explain the mode of action of a heterogeneous catalyst.
State one advantage of using a heterogeneous catalyst rather than a homogeneous catalyst.
Discuss two factors that need to be considered when choosing a catalyst for a process.
Radioactive waste must be disposed of with care.
State what is meant by the term high-level radioactive waste.
(i) Explain why high-level waste should not be disposed of by landfill or incineration.
(ii) State the name of one method of disposal used for high-level waste and explain why such a method is better than landfill and incineration.
The main ore used to produce aluminium by electrolysis is bauxite. Bauxite is mainly aluminium hydroxide, and contains iron(III) oxide and titanium(IV) oxide as impurities.
Explain how pure aluminium oxide is obtained from bauxite.
Explain why sodium hexafluoroaluminate, \({\text{N}}{{\text{a}}_{\text{3}}}{\text{Al}}{{\text{F}}_{\text{6}}}\), (cryolite) is added to the aluminium oxide before electrolysis takes place to produce aluminium.
State the half-equations for the reactions taking place at the positive and negative electrodes during the production of aluminium by electrolysis.
Positive electrode (anode):
Negative electrode (cathode):
Before the introduction of the electrolytic method by Hall and Héroult in the 1880s it was very difficult to obtain aluminium metal from its ores. Suggest one way in which it was achieved.
The worldwide production of aluminium by electrolysis makes a significant impact on global warming. Suggest two different ways in which the process increases the amount of carbon dioxide in the atmosphere.
One type of molecular vibration that occurs when \({\text{C}}{{\text{O}}_{\text{2}}}\) molecules are exposed to IR radiation is illustrated in the diagram below.
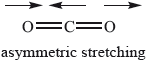
Identify two other types of molecular vibrations that occur when \({\text{C}}{{\text{O}}_{\text{2}}}\) molecules are exposed to IR radiation. Illustrate your answer with appropriate diagrams.
Climate change is a current global topic of debate.
Water and carbon dioxide are greenhouse gases present in significant quantities in the atmosphere. Identify one other greenhouse gas and its source.
Suggest the two factors that influence the relative greenhouse effect of a gas.
Describe how the greenhouse effect causes the atmosphere of the Earth to increase in temperature.
Identify one greenhouse gas other than \({\text{C}}{{\text{O}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\) and suggest a significant source.
The temperature of the Earth’s surface is currently increasing. Many scientists attribute this to an increase in the levels of greenhouse gases in the atmosphere as a result of human activity.
Explain how the interaction of greenhouse gases in the atmosphere with radiation could lead to an increase in the temperature of the Earth’s surface.
Suggest why carbon dioxide is the greenhouse gas most frequently connected with the effect of human activity.
Other than carbon dioxide and water, identify one other greenhouse gas and state its source.
Disposal of radioactive waste is a major ecological concern.
(a) State one source of low-level radioactive waste and one source of high-level radioactive waste.
Low-level waste:
High-level waste:
(b) Consider the following types of radioactive waste.
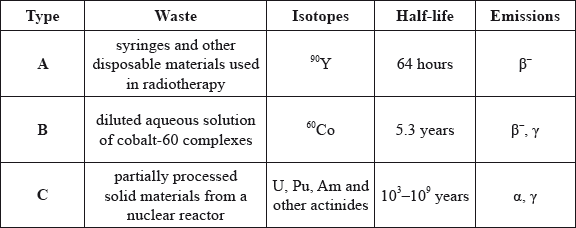
Identify which method can be used for the disposal of radioactive wastes A, B and C.
(i) Vitrification followed by long-term underground storage:
(ii) Storage in a non-shielded container for two months followed by the disposal as normal (non-radioactive) waste:
(iii) Ion-exchange and adsorption on iron(II) hydroxide, storage in a shielded container for 50 years, then mixing with concrete and shallow land burial:
“Oil should not be used as a source of energy because it has more important uses.” Suggest two arguments that support the continued use of oil as an energy source, and two against.
Vegetable oils, such as that shown, require conversion to biodiesel for use in current internal combustion engines.
State two reagents required to convert vegetable oil to biodiesel.
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
Determine the specific energy, in kJ\(\,\)g−1, and energy density, in kJ\(\,\)cm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 g\(\,\)cm−3; Molar mass = 299 g\(\,\)mol−1;
Enthalpy of combustion = 12.0 MJ\(\,\)mol−1.
Biofuels are renewable energy sources derived mainly from plants.
State the equation for the complete transesterification of the triglyceride given below with methanol.
Outline why the fuel produced by the reaction in (a) is more suitable for use in diesel engines than vegetable oils.
Suggest why the temperature decrease of the Earth’s surface after sunset is less when the weather is cloudy than when there are no clouds.
Increasing concentrations of greenhouse gases are considered to cause global warming. Ozone depletion is another environmental concern.
Identify a gas that is both a greenhouse gas and a cause of ozone depletion.
Atmospheric carbon dioxide and aqueous carbon dioxide in the oceans form a heterogeneous equilibrium.
Explain the effect of increasing concentrations of atmospheric carbon dioxide on the pH of the oceans, including an equation in your answer.
Explain what occurs at a molecular level during the absorption of infrared (IR) radiation by the sulfur dioxide molecule, \({\text{S}}{{\text{O}}_{\text{2}}}\).
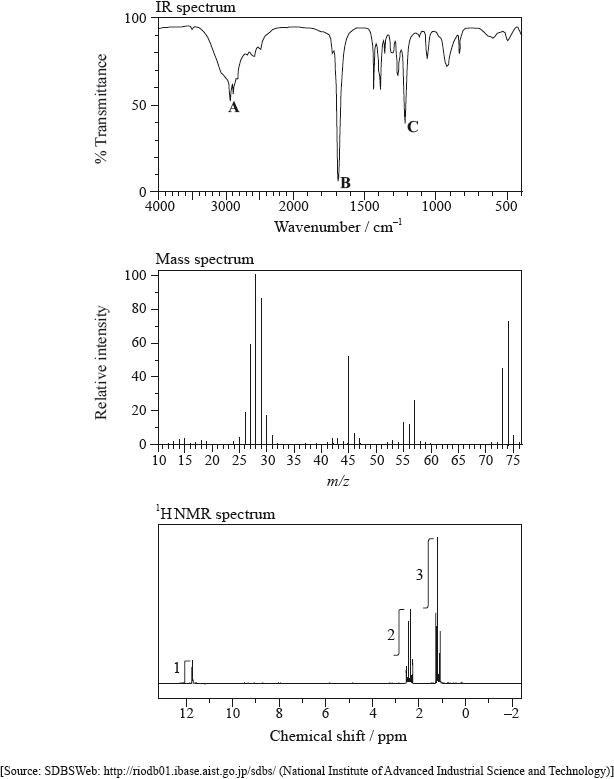
Consider the IR spectra of the following three compounds.
\[\begin{array}{*{20}{l}} {{\text{A}} = {\text{C}}{{\text{H}}_{\text{3}}}{{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{3}}}{\text{COOH}}} \\ {{\text{B}} = {\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}} \\ {{\text{C}} = {{{\text{(C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{3}}}{\text{COH}}} \end{array}\]
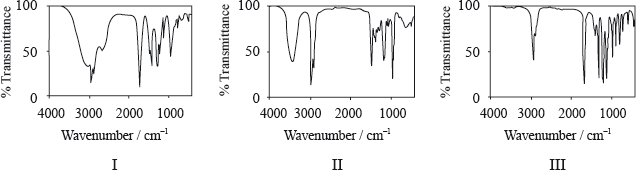
Determine which IR spectrum corresponds to each compound A, B and C. Explain your reasoning. IR data can be found in Table 17 of the Data Booklet.
There has been a shift in the use of crude oil (petroleum) away from its use as an energy source and towards its use as a chemical feedstock.
Suggest two reasons for this shift.
A lot of feedstock is used in the production of plastics. Discuss two advantages and one disadvantage of using plastic for packaging instead of cardboard.
Two advantages:
One disadvantage:
Fusion and fission reactions are important nuclear reactions.
Curium, \({}^{240}{\rm{Cm}}\), was synthesized by bombarding thorium nuclei, \({}^{232}{\rm{Th}}\), with carbon-12 nuclei. State a balanced equation for this reaction.
Uranium-235 has a half-life of 7.038×108 years.
(i) Determine the time required for the mass of \({}^{235}{\rm{U}}\) in a sample originally containing 1.000 g of \({}^{235}{\rm{U}}\) to decrease to 0.125 g.
(ii) Outline why products of the fission of uranium-235 must be disposed of carefully.
Outline why an element such as thorium, Th, usually undergoes nuclear fission, whereas helium, He, undergoes nuclear fusion.
Infrared (IR) spectroscopy is widely used as a technique in analytical chemistry.
The IR spectrum, mass spectrum and \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum of an unknown compound, X, of molecular formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\) are as follows.
Explain what happens at a molecular level during the absorption of IR radiation by carbon dioxide, CO2.
(i) Identify the bonds responsible for the peaks A, B and C in the IR spectrum of X.
A:
B:
C:
(ii) In the mass spectrum of X, deduce which ions the m/z values at 74, 45 and 29 correspond to.
m/z = 74:
m/z = 45:
m/z = 29:
(iii) Identify the peak at 11.73 ppm in the \(^{\text{1}}{\text{H}}\,{\text{NMR}}\) spectrum.
(iv) Deduce the structure of X.
The greenhouse effect maintains the Earth’s average temperature at a habitable level. The components of the Earth’s atmosphere responsible for this effect are called greenhouse gases.
(a) Major greenhouse gases are water vapour and carbon dioxide. State two other greenhouse gases.
(b) Describe how greenhouse gases cause the greenhouse effect.
(c) Discuss three possible implications of global warming on world food production.
Vegetable oils can be used as a source of energy.
State the structural feature of chlorophyll that enables it to absorb visible light.
Vegetable oils are too viscous for use as liquid fuels. Describe, using an equation, how a vegetable oil, such as that shown, is converted to oils with lower viscosity by reaction with methanol, CH3OH.
The diagrams below show a hydrogen-oxygen fuel cell with an alkaline electrolyte and a lead-acid battery (accumulator).
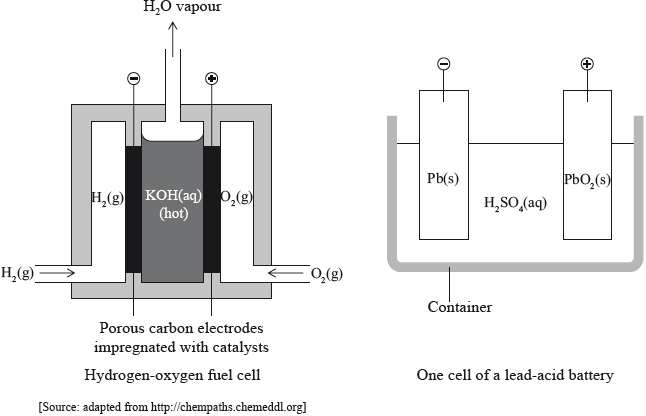
Discuss one advantage and one disadvantage for both fuel cells and lead-acid batteries.
Vegetable oils and diesel fuel have similar energy content but vegetable oils are not usually used as fuels in internal combustion engines.
Transesterification reactions allow waste cooking oils to be converted to biofuels. Identify a reagent and catalyst required for this conversion.
Reagent:
Catalyst:
Deduce the equation for the reaction that occurs assuming that the vegetable oil has the formula drawn below.
Scientists around the world conduct research into alternatives to fossil fuels. Suggest why collaboration is important.
Catalytic cracking uses heterogeneous catalysts.
The initial products of the fractional distillation of oil often undergo cracking. This can be carried out in a number of ways. State the major reason for choosing each of the following techniques.
Catalytic cracking:
Thermal cracking:
Steam cracking:
Explain how these differ from homogeneous catalysts.
Identify one disadvantage of using heterogeneous catalysts.
Many of the compounds produced by cracking are used in the manufacture of addition polymers. State the essential structural feature of these compounds and explain its importance.
The polymers often have other substances added to modify their properties. One group of additives are plasticizers. State how plasticizers modify the physical properties of polyvinyl chloride and explain at the molecular level how this is achieved.
The combustion of fossil fuels produces large amounts of CO2, a greenhouse gas.
The diagram below illustrates a range of wavelengths in the electromagnetic spectrum.
Synthesis gas, or syngas, mainly composed of CO(g) and H2(g), is an alternative form of fuel. It can be produced by coal or biomass gasification, passing steam over the source material in a low oxygen environment.
Identify which region, A or B, corresponds to each type of radiation by completing the table.
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) \( \rightleftharpoons \) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
Suggest an equation for the production of syngas from coal.
The Fischer-Tropsch process, an indirect coal liquefaction method, converts CO(g) and H2(g) to larger molecular weight hydrocarbons and steam.
Deduce the equation for the production of octane by this process.
Suggest a reason why syngas may be considered a viable alternative to crude oil.
There are many sources of energy available.
State one advantage and one disadvantage for each energy source in the table.
Calculate the specific energy of hydrogen, stating its units. Refer to sections 1, 6 and 13 of the data booklet.
Hydrogen has a higher specific energy than petrol (gasoline) but is not used as a primary fuel source in cars. Discuss the disadvantages of using hydrogen.